In the realm of pharmaceuticals, packaging is more than just a vessel for transport or storage. It serves as the first line of defence in ensuring the safety and efficacy of drugs.
Among the myriad of packaging requirements, child resistant packaging stands out as a critical component, especially in safeguarding our youngest and most vulnerable demographics. But what does this mean for the pharmaceutical industry, and how do legal implications shape packaging practices? In this post, we’ll explore these questions, offering insights into the regulatory landscape and its impact on the sector.
Understanding Child Resistant Packaging
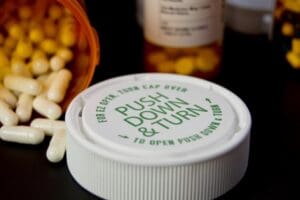
is designed to prevent children from accessing potentially harmful substances while allowing adults to open them without undue difficulty. This type of packaging is essential for products like medications, household chemicals, and certain cosmetics. The challenge lies in balancing accessibility for adults with safety for children, a task that requires meticulous design and manufacturing processes.
In the pharmaceutical industry, child resistant bottles and caps are commonly employed to achieve this balance. These components often feature push-and-turn mechanisms or squeeze-and-twist designs that are simple for adults to operate but difficult for young children to manipulate. The integration of these elements into packaging lines not only involves technical expertise but also adherence to stringent regulatory standards.
Impact on Pharmaceutical Companies
For pharmaceutical companies, child-resistant packaging involves more than just meeting regulatory requirements, it requires significant investment in machinery, design, and testing, which can be challenging, especially for smaller companies. However, the long-term benefits are substantial.
Staying compliant with these regulations means that pharmaceutical companies must integrate quality control measures throughout their supply chain. This can include continuous monitoring of production, quality assurance audits, and partnering with certified packaging suppliers. Failure to comply with child-resistant packaging regulations can result in severe consequences, including product recalls, fines, and reputational damage. Companies must, therefore, maintain up-to-date knowledge of changing legal requirements and emerging best practices.
Innovations in Child-Resistant Packaging Technologies
Advancements in technology have led to new innovations in child-resistant packaging, enhancing both safety and user experience. Smart packaging solutions, for example, are gaining traction in the industry. These may include digital features like embedded QR codes that provide instructions on how to open the packaging correctly or smart caps that only unlock when paired with a specific smartphone app. These technologies are particularly beneficial in preventing accidental ingestion by children while ensuring convenience for authorised users.
Moreover, there has been a shift towards the use of sustainable and eco-friendly materials in child-resistant packaging. Biodegradable plastics, recycled materials, and other eco-conscious options are being explored to address environmental concerns. The challenge lies in ensuring that these materials maintain the same level of child resistance as traditional packaging options. Manufacturers are investing in research and development to strike a balance between eco-friendliness and compliance with child safety standards.
Accessibility and Inclusive Design Considerations
As the demographic profile of consumers changes, inclusive design has become an important aspect of child-resistant packaging. Packaging solutions must not only keep children safe but also be accessible to older adults and individuals with physical limitations or disabilities. For example, arthritis-friendly caps and designs that require minimal strength or dexterity are becoming more common. Inclusive design ensures that pharmaceutical packaging serves the needs of all users, fostering greater compliance with medication usage and enhancing overall user experience.
To achieve this, packaging designers often collaborate with human factors experts to study user interactions and develop prototypes that can be tested by diverse groups. Feedback from these tests helps refine the packaging, ensuring it meets both child safety and accessibility standards. Such user-centred approaches are becoming increasingly significant, as regulators and consumers alike prioritise designs that do not exclude vulnerable populations.
Global Market Dynamics and Competition
Global competition has driven the need for differentiation, leading companies to adopt advanced manufacturing techniques and materials that support sustainability and safety. For example, smart polymers that change colour when tampered with or indicate expiration dates are becoming part of the competitive landscape. Such innovations can help companies comply with international standards and appeal to markets that value high-quality, secure, and sustainable packaging solutions.
Impacts On the Industry
The legal requirements for child-resistant packaging in the pharmaceutical industry impact every stage, from design and testing to manufacturing and marketing. Understanding these regulations is essential for companies to protect consumers, ensure compliance, and safeguard brand reputation. Consulting with legal or regulatory experts can help companies develop compliant packaging solutions that meet safety standards and adapt to evolving consumer needs. With proper guidance and a commitment to innovation, the pharmaceutical industry can confidently navigate regulatory demands while focusing on consumer safety.